[TOC]
Main buffers
1. bicarbonate
2. hemoglobin
3. phosphate
4. bone
Each hydrogen sponge has an associated Ka which indicates its ability to absorb hydrogen
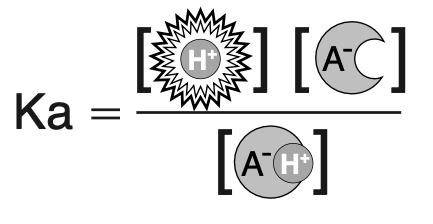
1. Ka is the ratio of unbound hydrogen and unbound sponge to bound hydrogen and sponge at equilibrium
2. A poorly absorbent sponge has a high Ka: the majority of hydrogen is free
3. A highly absorbent sponge has a low Ka: the majority of hydrogen is bound
4. pKa is the negative log of Ka
1. High pKa = hydrogen is mostly bound
2. Low pKa = hydrogen is mostly free
Bicarbonate Buffer
- Primary hydrogen buffer is bicarbonate
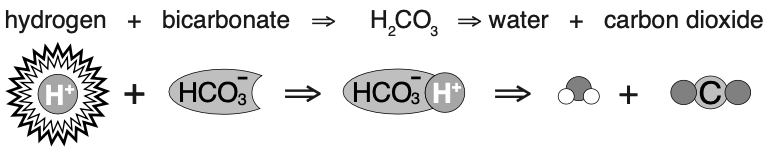
- presence of excess hydrogen ion causes the consumption of bicarbonate and the formation of H₂O and CO₂
- presence of diminished hydrogen ion causes formation of hydrogen and bicarbonate and consuming CO₂
- Excess bicarbonate formed must be excreted by the kidney
- H₂O + CO₂ <-> H2CO3 <-> H+ + HCO3-
- H2CO3 is rapidly broken down by carbonic anhydrase so dropped from equation
- H₂O + CO₂ <-> H+ + HCO3-
- Ka of bicarbonate is the ratio of products (H+ & HCO3-) to the reactants (H₂O & CO₂)
- Concentration of H₂O does not change i.e. its a constant
- Ka = (H+ x HCO3-)/ CO₂
- This Ka has a value of 800 nanomol/L
- pKa has a value of 6.1
Hemoglobin Buffer
- The terminal imidazole groups also are available to buffer H+
- Oxygenated hemoglobin is a stronger acid but weaker buffer than unoxygenated hemoglobin
- Thus the buffering capacity of venous blood is greater than that of arterial blood